



Mycoplasma pneumoniae (M. Pneumonia, MP) is the pathogen of human mycoplasma pneumonia. The pathological changes of mycoplasma pneumonia are mainly interstitial pneumonia, sometimes complicated by bronchial pneumonia, which is called primary atypical pneumonia. Worldwide, multiple studies have shown that the annual MP infection rate is 9.6% to 66.7%, the infection rate during non-epidemic period is 10% to 20%, and MP accounts for more than 30% of pediatric pneumonia during epidemic period. There are approximately 156 million children with pneumonia each year, of which 151 million children with pneumonia occur in developing countries and are the leading cause of death for children under 5 years of age, accounting for 19% of the total number of deaths. It is also the main cause of death among adults, especially among the elderly.
Mainly infected by droplets, the incubation period is 2 to 3 weeks, and the incidence is the highest among adolescents. The clinical symptoms are mild, or even asymptomatic at all, and if there are only general respiratory symptoms such as headache, sore throat, fever, cough, etc., but there have been individual death reports. It can happen all year round, but mostly in autumn and winter.
|
Mycoplasma pneumoniae nucleic acid detection kit (PCR-fluorescent probe method) |
|
|
Sensitivity |
5.0x103copies / mL |
|
Linear range |
1.0x104copies / mL ~ 1.0x 109copies / mL |
|
Accuracy |
With reference to international reference products, the coincidence rate of the test results is 100% |
|
Precision |
Coefficient of variation within and between batches CV≤5% |
|
Specificity |
100% specificity, no cross reaction with Streptococcus pneumoniae, Mycobacterium tuberculosis, Epstein-Barr virus, pertussis virus, adenovirus, respiratory syncytial virus, etc. |
|
Anti-interference |
Mucin, blood, pus, erythromycin, chloramphenicol did not interfere with the test results |
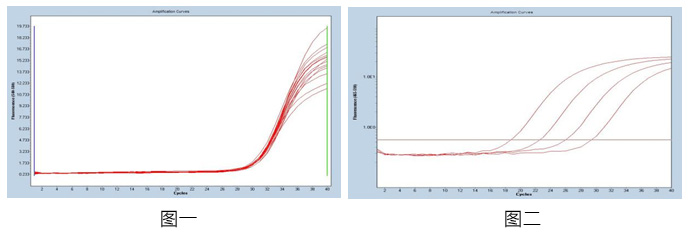
Figure 1: Using 1x104copies / mL reference as a template, repeat the 20-well test in the same batch, calculate the coefficient of variation of the CT value in the batch, and the CV value is ≤5%, with good consistency.
Figure 2: For the reference products in the concentration range of 107-104 copies / mL, the product amplification curve is smooth and complete, and the correlation coefficient between different gradients is R> 99%, and the correlation is good.